The evolving impacts of refrigerants over time

Abstract
The first series of synthetic refrigerants – CFCs and HCFCs, based on Midgley’s work – depleted the ozone layer, resulting in the Montreal Protocol in 1987. Their successors – HFCs – are now under regulation under the same protocol because of their significant global warming potential (GWP). Chemical companies have since developed a new series – HFOs – which have come under scrutiny for breaking down easily into different, more stable compounds such as trifluoroacetic acid (TFA) that come with their own problems. This paper argues that, even though such substances might be listed as having a low GWP, the trouble with GWP’s definition is that it doesn’t take into account the harms caused by the compounds these substances break down into.




Thomas Midgley Jr and Frédéric Jean Edmond Swarts (left to right)
A difficult legacy
Frédéric Jean Edmond Swarts (September 2, 1866 – September 6, 1940) was a Belgian chemist and professor in civil engineering at the University of Ghent who is credited with developing the first chlorofluorocarbon (CFC); the Swarts process named after him is used when producing fluorinated gases. However, Thomas Midgley Jr (May 18, 1889 – November 2, 1944) popularised the invention of Freon (R12), which laid the foundations for future synthetic refrigerants. Midgley was an innovative gentleman who created leaded petrol for cars, another invention that is considered problematic today. In an attempt to prove to journalists that leaded petrol was safe, Midgley poured it over his hands and inhaled deeply; shortly afterwards, he fell ill, before contracting polio in 1940. In a macabre twist that serves as the perfect metaphor for the legacy of both Freon and leaded petrol, Midgley invented a machine to lift him in and out of his wheelchair, only to get caught in the machine and die of strangulation.
The development of refrigerants
The first series of refrigerants was based on methane (R50 or CH4), which has four hydrogen atoms that can be exchanged with halogens, such as fluorine, chlorine, bromine, and iodine. This can be organised as shown in the grid in Figure 3iii.
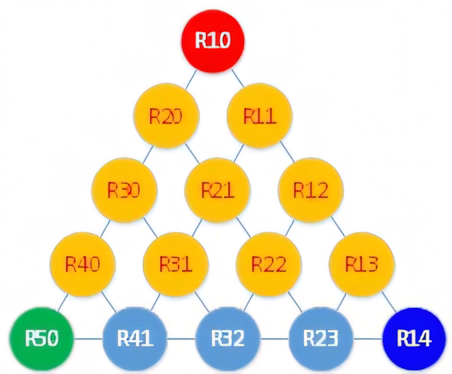

Figure 1: Methane series of halogenated variants thereof
The most popular refrigerants before the adoption of the Montreal Protocol in 1987 were R12, R22, and the blend R502 consisting of R22 and R115, the latter being a fully halogenated member of the ethane family.
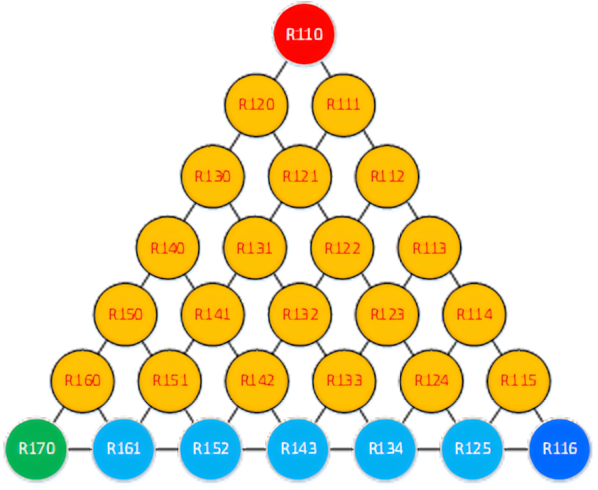
Figure 4: The ethane series of refrigerants. R134a became one of the three most used refrigerants ever
Ethane (R170 or C2H6) has two carbon atoms. Its higher number of hydrogen atoms allows for more variants, as shown in Figure 4.
The most used refrigerant of the ethane family became R134a, which is a variant of R134.
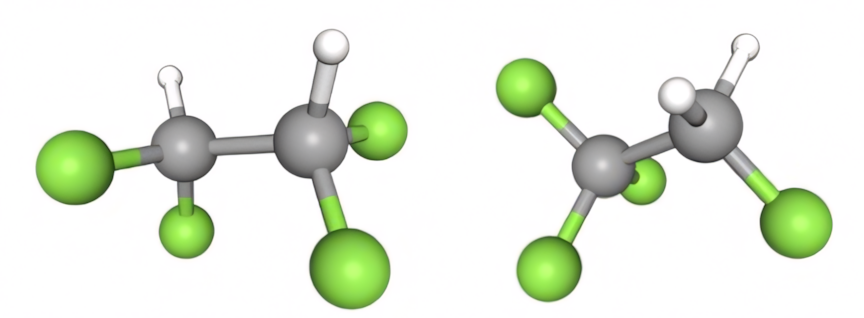
Figure 5: R134 (left) and R134a (right)
The light blue fluids in both family trees are the HFCs that took over the market when CFCs were banned. They contain no chlorine and have no ozone depletion potential (ODP). The strength of the first CFCs was their stability. Unfortunately, this meant a very long atmospheric lifetime, which equated to a high global warming potential (GWP). Despite their lack of ODP, HFCs still have significant GWP, which is why they have now been regulated globally.
The dark blue circle represents PFC fluids with extremely long atmospheric life; the closer you get to this point, the longer the expected life becomes, and the higher the GWP of the refrigerant. R23 and R125 have very high GWP because they are stable and have long atmospheric lives.
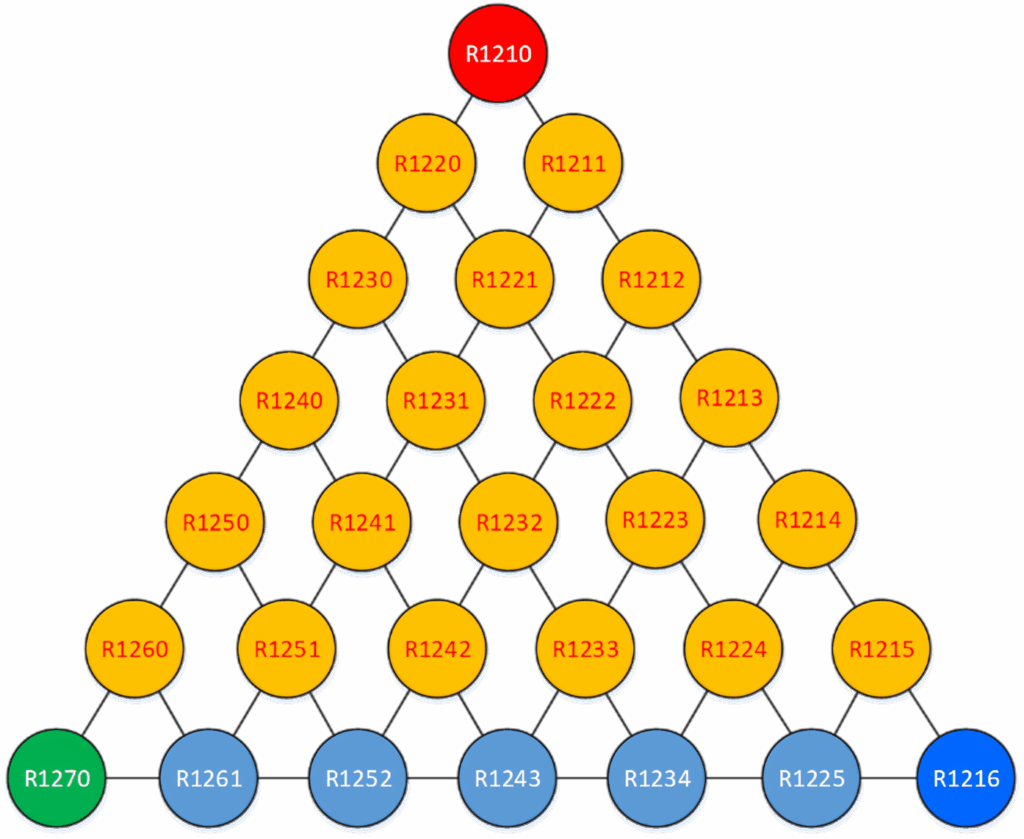
Figure 6: Propene family tree. The best known here are the R1234 series. One also sees that R1233 and R1224 are in yellow, which here indicates they have an ODP.
Propene (R1270 or CH₃CH=CH₂) has a double bond, making it less stable in the atmosphere, like the halogenated family members; its atmospheric life is hours to a few days. At first glance, this means a low GWP of 2, but unfortunately the reality isn’t that simple.
Figure 7: The most used of the R-1234 variants is the R1234yf, commonly used in car AC systems.
As the base hydrocarbon becomes longer, the number of molecule variants increases, but the chlorine atoms limit the number of molecules that can be used. Here we’re focusing on the light blue at the lower end of Figure 6.
Refrigerant breakdown
Basic chemistry dictates that synthesised molecules can and will degrade into different breakdown products that become more stable than the initial industrialised molecule. In the case of the R1270 series, the number of valuable molecules has increased from 3 to 5; if we go to the butene series with four carbon atoms, the number of HFCs becomes 7. However, they have one problem in common: their breakdown products are an environmental challenge.
Table 1 several of the breakdown products are strong acids
Table 1 shows some standard and breakdown products for comparison, namely trifluoroacetic acid (TFA) and hydrofluoric acid (HF), which are relatively strong acids – especially TFA. These breakdown products have been known for a while, but not necessarily respected.
Carbonyl fluoride is another toxic standard breakdown product of many fluorinated working fluids. Its lethal toxicity level for humans is about 300ppm when inhaled, but even below these levels it can have severe health implications. In the Gestis-ILV app (free to download), the eight-hour exposure limit is set at 2ppm; for 15 minutes of exposure, which is not easy to measure, that exposure limit is 4–5ppm. Carbonyl fluoride is a powerful acid with a pKa of -6.5, which is stronger than hydrochloric acid (pKa -5.9).
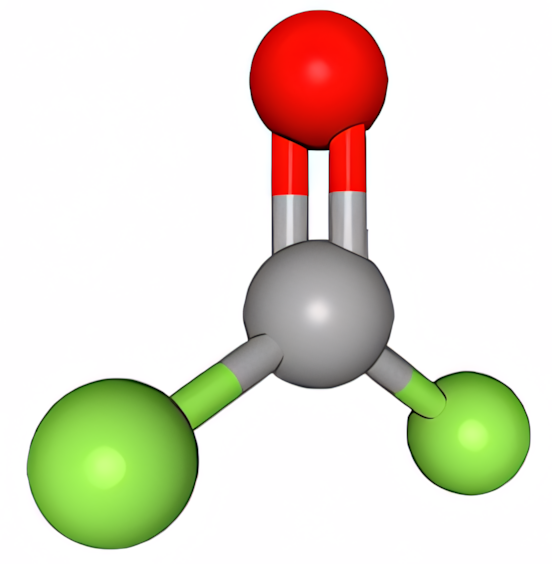


Figure 8: Carbonyl fluoride
Figure 9: Perfluorooctanoic acid (PFOA)
PFAS and TFA
Per- and polyfluoroalkyl substances (PFAS) are known as “forever chemicals” because of their persistence in the environment and their tendency to bioaccumulate. They have been used in a wide range of applications because of their resistance to stains, water, grease and heat.
Perfluorooctanoic acid (PFOA) and perfluorooctane sulphonic acid (PFOS) were among the first PFAS substances used, and they were used very widely. They are now banned in many parts of the world after they were discovered to be a significant health threat. However, despite being banned, both of these substances are s still routinely present in test samples of human blood, water, soil, and food, because of their tendency to bioaccumulate.
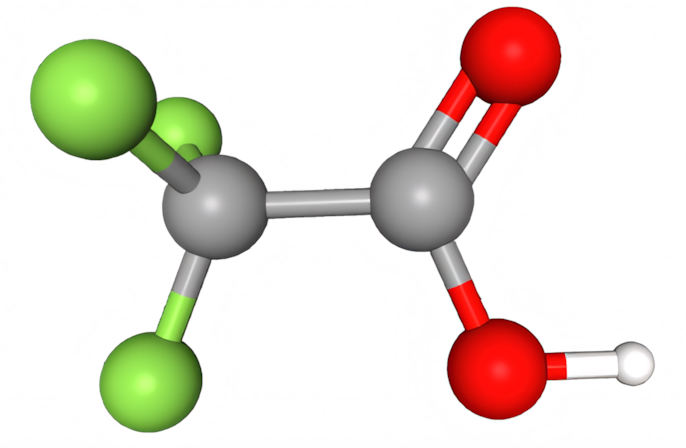
Figure 10: Trifluoroacetic acid
TFA is one such bioaccumulating substance that has caused public concern, especially because of its link to refrigerants. Most refrigerants break down, but some HFOs produce more TFA than previously used HFCs. Data for Figure 11 can be found in a report produced for the German Umweltbundesamt (UBA) (Germany’s equivalent of the EPA).
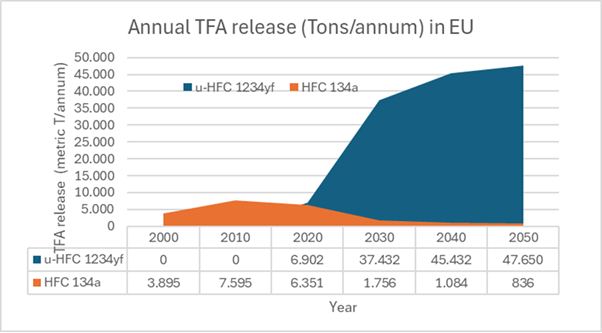
Figure 11: It has long been known that R134a can break down into TFA. R1234yf produces more, and with the anticipated growth in consumption, the challenges increaseiv.
It’s not only refrigerants that break down into TFA or other forms of PFAS; such substances are also used in products such as electronics, furniture, and outdoor clothing because of their water- and oil-repelling properties. However, in many of these other cases, PFAS substances end up in landfill, which can take years to leak into the environment. Refrigerants, on the other hand, are directly emitted into the atmosphere and quickly form PFAS/TFA.
Here, it is important to understand that the atmospheric life of a refrigerant determines the GWP because the breakdown products are currently not included in the calculation. Hence, the interest in double-bond fluids from the manufacturer’s side.
The alternatives
There are good and efficient alternatives to fluorinated substances, and competent, well-trained engineers and technicians can work with them safely.
These alternatives are:
- Ammonia (R717 or NH3)
- Carbon dioxide (R744 or CO2)
- Hydrocarbons – a series including ethane, iso-butane, propane, iso-pentane, etc.
- Water (R718 or H2O)
- Air (*R729)
Ammonia
Ammonia is often called the workhorse of industrial refrigeration. It has been in use since the 1850s, initially in absorption systems and later in Carnot cycles. Despite its toxicity and other limitations, the industry has developed ways to work safely with ammonia and design systems that are compatible with NH3’s pressure and material requirements. Ammonia competed against the first CFCs – especially HCFC-22 – as well as HFCs, but it remains a dominant working fluid, especially in industrial refrigeration, where its GWP and ODP of 0 make it attractive.
Carbon dioxide
Carbon dioxide was first used as a refrigerant in freezing applications in 1869v. It was highly successful, especially onboard ships, where freezing was an effective alternative to salting meat, especially on long journeys. In the early 1900s, CO2 was considered more dangerous than ammonia, as the latter could be smelt to detect a leak. The technology used at the time needed development to become leak-tight enough to handle the higher pressures that CO2 has, which was another challenge.
With the introduction of CFCs in the 1940s, CO2 quickly disappeared even from ships; its last recorded installation was in 1955. Thanks to its GWP of 1 and its ODP of 0, CO2 was reintroduced in the early 1990s by several scholars, most prominently by Prof Gustav Lorentzen from Trondheim, Norway. CO2 refrigeration is now a fast-growing technology that is replacing fluorinated gases in many applications, especially supermarkets.
Hydrocarbons
Standards for the use of hydrocarbons have changed over the years, reflecting changing attitudes towards them. Hydrocarbon refrigerants were, for a period before the launch of the CFCs in the 1940s, seen as the safer choice than ammonia and CO2, despite their flammability. When CFCs were dominant, it seemed there was no reason to use flammable refrigerants, although hydrocarbons continued to be used in specialised processes such as petrochemical applications.
On the whole, hydrocarbons were forgotten for several decades, and standards were gradually adjusted so that their use in practice was more or less impossible until their recent revival. Shortly after the signing of the Montreal Protocol, reports of experimental setups with propane and iso-butane showed these fluids worked very well for many applications.
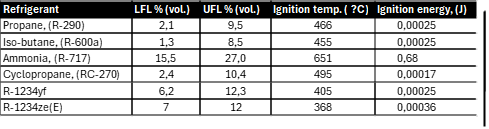
Table 2 Properties of some flammable refrigerants
Hydrocarbon refrigerants, such as propane (R290) and isobutane (R600a), are classified as A3 refrigerants, meaning they are low in toxicity but highly flammable. They are considered safe when handled correctly.
Table 2 shows the combustible properties of some working fluids. The autoignition temperature of hydrocarbons is higher than that of fluorinated substances in the A2L safety class. Despite their flammability, they offer several benefits:
- Eco-friendly: Hydrocarbons have zero ozone depletion potential (ODP) and very low global warming potential (GWP), making them environmentally friendly alternatives to traditional refrigerants.
- Efficiency: Their excellent thermodynamic properties can increase energy efficiency in cooling systems.
- Versatility: Hydrocarbons can be used in various applications, including domestic and commercial refrigeration, air conditioning, and heat pumps.
To mitigate the risks of their flammability, safety measures – such as proper system design, adherence to safety standards (like ISO 5149/EN 378, IEC 60079-10-1, and IEC 60335), and professional handling by “competent persons” – are crucial.
Water and air
Water and air are among the most used heat transfer media. However, water has also been considered as a primary refrigerant in certain studies. It has proven a viable solution, especially in closed loops with clean water, although certain contaminants still present a challenge. Its capacity and efficiency are high, especially in the temperature range heat pumps typically operate at. However, the swept volume required in a water vapour compressor is very high in normal air conditioning and operation conditions. As such, most projects using H2O are using turbo machines with high rotational speed to keep down the dimensions of the compressorvi.
Air is also used as a working medium, although not in a classic Carnot cycle. The air cycle is based on the Jule-Thomsen effect, and high-speed turbo machines are typically required. Air is typically used in ultra-low-temperature applications like medicine, with cooling needs at -80 ˚C to -150˚Cvii. An air cycle has also been proposed for air conditioning, but there is little published work into the effectiveness of this idea.
The future of the industry
It’s difficult to imagine a future for any fluorinated refrigerants given the environmental challenges stemming from their breakdown when released to the atmosphere. Releases cannot be avoided; even household appliances like fridges often have high leak rates over their lifetime.
Synthetic refrigerant has been recycled for decades, but the real impact of this is debatable. The European Environmental Agency (EEA) produces annual reports on F-gas use in Europe every year showing that only a marginal amount of refrigerant is recycled, and the recycled material will most likely end up in the air somedayviii. Natural refrigerants also leak, but their impact is negligible. Only catastrophic releases are noticed, but that goes for all refrigerants.
Standards and guidelines, training, and education according to ISO 22712 are all crucial for ensuring long, trouble-free system lifespans and the highest possible energy efficiency throughout the service life of the equipment. This applies to all stages of the process, including design, product selection, building the system, assembling and installing the system, service, maintenance, decommissioning, and leak checking.
The refrigeration and heat pump industry faces a challenging future when selecting refrigerants. Aside from the environmental issues discussed above, the supply of fluorine in a reasonable and competitive quality is limited, and what will be left for future generations is low-level quality, making the production of acid-grade HF as a base stock for the production of fluorinated gases less competitive. Reports from the EU show prices increasingix, and USGS reports show that the amount left in the world at a reasonable quality could be exhausted quickly at current consumption ratesx.
Conclusion
Thomas Midgley Jr and those who followed in his footsteps might not have understood the full ramifications of their inventions, but we do, and it’s our responsibility to deal with their legacy. The only way forward, and the only decent way to go for the sake of coming generations, is to use natural refrigerants, as described in this paper.
About the Author

Alexander Cohr Pachai, AM.AIRAH, has been active in refrigeration, air conditioning and heat pumps since 1978. His apprenticeship was successfully ended by installing a heat pump in 1981. Since then, Alex has held a number of senior positions within the industry and is now an independent consultant working out of Denmark. Alex was the keynote speaker at AIRAH’s 2025 Refrigeration Conference in Melbourne.
References
[1] https://pubs.acs.org/doi/10.1021/ed032p301#Abstract
[1] Prisco, Jacopo, Once celebrated, an inventor’s breakthroughs are now viewed as disasters – and the world is still recovering. Thomas Midgley Jr.: The man who almost destroyed the planet (twice) | CNN
[1] Pachai, A.C.: Efficient systems compared in different climates. 50th KgH congress and exhibition, Belgrade 2019.
[1] Reducing the input of chemicals in to waters; trifluoroacetate (TFA) as a persistent and mobile substance with many sources; Sources, input pathways, environmental contamination of TFA and regulatory approaches. Umwelt Bundesamt, November 2021. hgp_reducing_the_input_of_chemicals_into_waters_v2.pdf
[1] Williams, S., Bodinus, P.E.: The Rise and Fall of Carbon Dioxide Systems. ASHRAE JOURNAL, April 1999.
[1] Pachai, A.C., Madsbøll, H.: ICR Prague
[1] ᐉ Open cycle system • ULT Freezer Room Open Cycle • Open air cycle refrigeration system
[1] Sylvie Ludig, Wolfram Jörß, Victoria Liste, Peder Gabrielsen: ETC CM Report 2024/05 Fluorinated greenhouse gases 2024. ETC CM report 2024/05: Fluorinated greenhouse gases 2024 — Eionet Portal
[1] Reports – Fluorinated Greenhouse Gases – Climate Action [1] pp1802g.pdf

This article appears in Ecolibrium’s Spring 2025 edition
View the archive of previous editions
Latest edition
See everything from the latest edition of Ecolibrium, AIRAH’s official journal.





